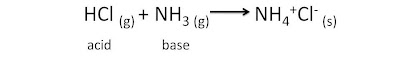The periodic table is a table of elements arranged in order of increasing proton number to show the similarities of the chemical elements with related electronic structures.
GROUPS
A vertical column of the periodic table containing elements with similar properties with the same number of electrons in the outer shell. They have an increasing number of inner shell as you descend the group.
There are 8 groups of elements.
The first column is called group 1 (alkali metals); the second group 2 (alkaline earth metals); and so on up to group 7 (halogens). The final column in the periodic table is called group 0 (inert gases or noble gases)
PERIODS
Horizontal rows of the periodic table. Within a period the atoms of all the elements have the same number of occupied shells but have an increasing number of electrons in the outer shell.
Between groups 2 and 3 is the block of elements known as transition elements
The periodic table can be divided into two by a blod line that starts beneath boron.
The elements to the left of this line are metals and those on the right are non metals.
The elements which lie on the line are called metalloids. These elements behave in some ways as a metal and in others as non metals.
GROUP 1 - the alkali metals
Consists of five metals lithium, sodium, potassium, rubidium, caecium and the radioactive element francium
lithium, sodium and potassium have the following properties:
They;
- are very reactive metals
- are good conductors of heat and electricity
- are soft metals, lithium is the hardest and potassium the softest.
- have low densities
- have shiny surfaces when freshly cut with a knife
- have low melting points
- burn in oxigen or air with characteristic flame colours
- react vigorously with water to give an alkali solution of the metal hydroxide plus hydrogen gas. Potassium is the most reactive followed by sodium and then lithium
- react vigorously with halogens such as chlorine to form metal halides eg. Sodium chloride
GROUP 2 - the alkaline earth metals
Consists of the five metals beryllium, magnesium, calcium, strontium and barium and the radioactive element radium.
Magnesium and calcium have the following properties.
- they are harder than those in group 1
- they are sivery-grey in colour when pure and clean. They tarnish quickly, however, when left in air due to the formation of metal oxides on their surfaces
- they are good conductors of heat and electricity
- they burn in oxygen or air with characteristic flame colours to form solid white oxides
- they react with water but do so much less vigorously than the elements in group 1
GROUP 7 - the halogens
Consists of the four elements flourine, chlorine, bromine and iodine, and the radioactive element astatine.
chlorine, bromine and iodine have the following properties:
- they are coloured and darken going down the group
- they exist as diatomic molecules eg. Cl2, Br2 and I2.
- they show a gradual change from a gas (Cl2), through a liquid (Br2), to a solid (I2).
- they form molecular compounds with other non-metallic elements eg. HCl
- they react with hydrogen halides, which dissolve in water to form acidic solutions - they react with metals to produce ionic metal halides eg. Iron (III) chloride
Colours of some halodens.
Chlorine - Pale green
Bromine - Red-brown
Iodine - Purple-black
Displacement Reaction
If chlorine is bubbled into a solution of potassium iodide, the less reactive halogen is displaced by the more reactive chlorine
2KI + Cl2 = 2KCl + I2
The halogens have many varied uses
· Floride is used in the form of florides in water and toothpastes it reduces tooth decay by hardening the enamel
· Chlorine is used to make PVC plastic as well as in household bleaches. It is also used to kill bacteria and viruses in drinking water
· Bromine is used to make disinfectants, medicines and fire retardants
· Iodine is used in medicines and disinfectants and also as a photographic chemical
GROUP 0 - the noble gases
Helium, neon, argon, krypton, xenon, and the radioactive element radon make up the noble gases
They are colourless gases